Engel Lab awarded R03 from NCI to study Beckwith-Wiedeman Syndrome
Award will support conformational, epigenetic and expression studies
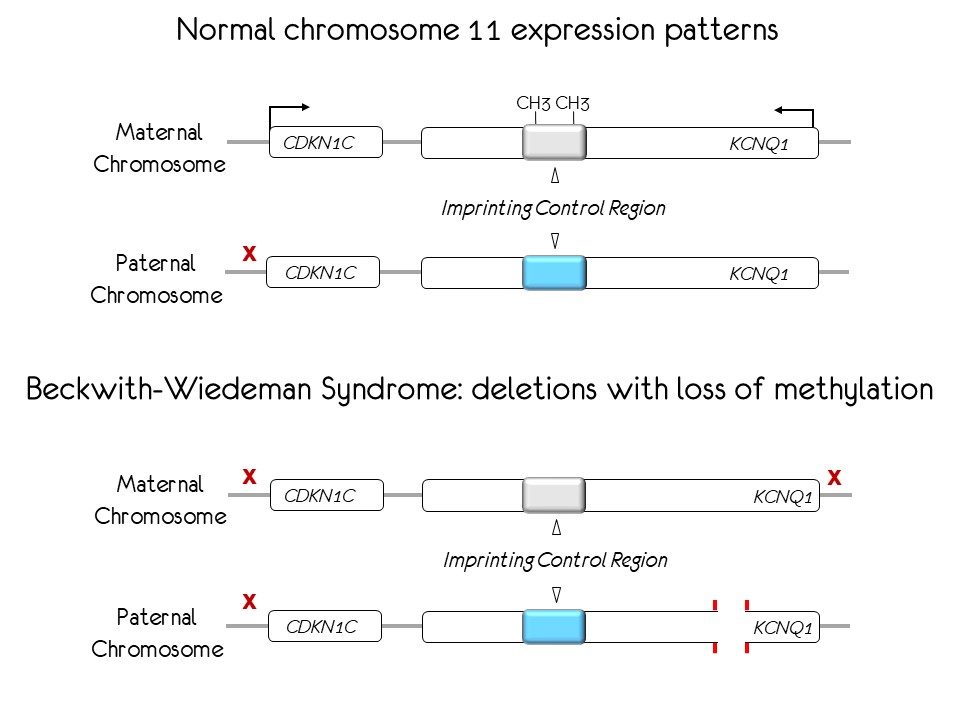 Image credit: Nora Engel
Image credit: Nora EngelAbstract
Beckwith-Wiedeman Syndrome (BWS) is a fetal overgrowth disorder which confers high risk for pediatric cancers. Loss of DNA methylation (LOM) in a key regulatory region of the KCNQ1 domain resulting in silencing of a critical cell cycle inhibitor, CDKN1C, occurs in a large group of patients. However, it is unknown how methylation is disrupted, how it affects other chromatin features and how it leads to tumor development. Thus, BWS provides a useful paradigm for addressing epigenetic dysregulation in cancer and offers an opportunity to test novel epigenetic therapeutic strategies. We will perform conformational, epigenetic and expression studies to determine the status of known promoters, enhancers and insulators and to identify novel regulatory sequences in the human KCNQ1 domain. We will also perform comparative analyses between normal fibroblasts and cells from BWS patients exhibiting LOM with co-occurring deletions. These comparisons will elucidate how LOM affects expression of critical genes in carcinogenesis and explain how the sequences spanned by patient deletions affect conformation and DNA methylation. We will engineer similar deletions in normal fibroblasts and epigenetically engineer normal cells to demethylate the DNA sequences affected in BWS. In addition, we will exploit epigenetic fusion proteins to rescue the epigenetic and transcriptional profiles of the patient cells. Because current epigenetic therapies act in a genome-wide manner, successful modification of specific loci with epigenetic editors portends a highly targeted therapy applicable to any cancer with deregulated gene expression.
Public Health Relevance
Beckwith-Wiedeman Syndrome (BWS) is a fetal overgrowth disorder which confers high risk for pediatric cancers. The steps that progressively disorganize the genes in the KCNQ1 region are not known. New technologies will be applied to cells from BWS patients, providing a useful paradigm for understanding the events that are misregulated in cancer and offering an opportunity to test novel epigenetic therapeutic strategies. We will perform conformational, epigenetic and expression studies to compare normal fibroblasts and cells from BWS patients. These comparisons will elucidate how expression of critical genes is disrupted. We will mimic alterations of patient cells in normal fibroblasts to determine the step-wise events that affect cell growth. In addition, we will exploit epigenetic fusion proteins to rescue the normal phenotype of the patient cells. Because current epigenetic therapies act in a genome-wide manner, successful modification of specific loci with epigenetic editors portends a highly targeted therapy applicable to any cancer with deregulated gene expression.